"For those patients who use these products, there is a potential risk of eye infections or related harm," the FDA said. "These products are intended to be sterile. Ophthalmic drug products pose a potentially heightened risk of harm to users because drugs applied to the eyes bypass some of the body’s natural defenses."
Brassica Pharma Pvt. Ltd. is voluntarily recalling the products with expiration date ranging from February 2024 to September 2025.
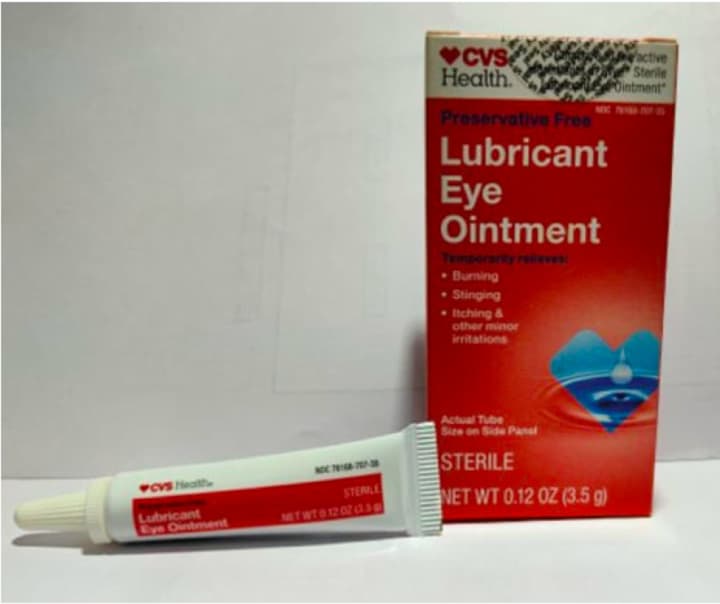
The recalled products, all in 3.5 gram packages, are:
- CVS Health Lubricant Eye Ointment, UPC code: 050428634141.
- Equate Lubricant Eye Ointment, UPC code: 681131395298.
- Equate Style Lubricant Eye Ointment, UPC code: 681131395304.
- Lubricant PM Ointment by AACE Pharmaceuticals, UPC code: 371406124356.
To view the package labels for the four products, check this link from the FDA.
To date, Brassica Pharma Pvt. Ltd., based in India, has not received any reports of adverse events related to this recall.
Click here to follow Daily Voice New Rochelle and receive free news updates.